Abstract
Introduction: Ponatinib (PON) is a third generation tyrosine kinase inhibitor (TKI) indicated in the treatment of CML-chronic phase (CP), accelerated phase (AP) and blast phase (BP) as well as in patients with the gatekeeper T315I mutation. TOPASE is the real-life observatory initiated in France with the participation of 42 CML centers, which represents one of the largest real-life ponatinib study to date in CML. We report here the updated data issued from the last pre-planned interim results of the 120 patients included in the study.
Methods and Aims: CML patients (pts) > 18 years old, with any stage of disease treated with PON for a period of less than 6 months or prospectively, were included in the study from 02/2018 to 12/2020 (ambispective study). The main endpoints were the evaluation of efficacy (proportion of CP-CML pts who achieve a major molecular response [MMR] or proportion of AP/BP pts who achieve a complete hematologic response [CHR]), as well as the safety profile of the use of PON. The final analysis study will be performed by the end of 2022 after 2 years of follow-up of the last patient included.
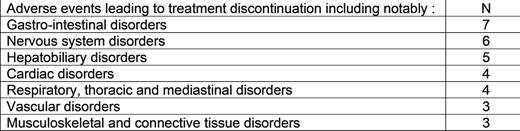
Results: Overall, 44.2% of pts were female and 55.8% males with median [Q1;Q3] age of 57.8 [44.8;69.1] years. One hundred and four (104, 86.7%) patients were in CP and 16 (13.3%) in AP/BP. The Sokal score at diagnosis was high in 29.1% of pts, intermediate and low in 24.1% and not available in 22.5 %. The number of previous TKI received was of: 2 in 49.2% of pts, 3 in 23.3%, 1 in 14.2%, 4 in 10.8%, 5 in 0.8% of pt. The 4 previous TKI received were: imatinib (75% of pts), dasatinib (71.7%), nilotinib (52.5%) and bosutinib (29.2%). The last TKI administered prior to inclusion was dasatinib (40.8% of pts), nilotinib (23.3%), bosutinib (21.7%), imatinib (10.8%) and ABL001 (1.7%). The main reason for the initiation of PON was "poor tolerance to previous therapies” (60.2% of pts) followed by "poor response to previous therapies” (25%) and "response enhancement” (8.3%). An ABL kinase mutation was detected in 31 (25.8%) pts at inclusion: 14 (11.7%) pts with a T315I mutation. At inclusion, 56 pts (46.7%) presented a cardiovascular (CV) history, notably 39 pts (32.5%) with a high blood pressure (HBP) history. At the time of the analysis, the median [Q1 : Q3] follow-up (FU) duration was of 18.2 months [6.3 ; 27.6] in pts in CP and 5.3 months [1.6 ; 13.3] in pts in AP/BP, respectively. The median [Q1 : Q3] treatment duration until stop or last FU was of 18.8 months [8.6 ; 28.5] in pts in CP. In CP-CML pts, the initiating dose was of 15 mg/day in 35.6 % of pts, 30 mg/day in 44.2% and 45 mg/day in 20.2%. Patients with AP/BP were mainly treated with 45 mg/day (62.5%) or 30 mg/day (25%). The analysis of the dose intensity (DI) in CP-CML pts shown that at month 3 (M3) visit the mean dose (± SD) was of 16.8 (± 5.5), 26.9 (± 8.9) and 35.8 (± 11.7) in the pts treated initially with 15, 30 or 45 mg/day respectively. Following M3 visit, the DI remained relatively stable in each cohort despite a steady decline in the number of pts per cohort at each visit. Among 70 out of 95 CP-CML pts and 9 out of 14 AP/BP who were non-responders (no MMR for CP-CML and no CHR for AP/BP) at initiation time, 60.0% and 66.7% achieved at least once the primary end-point or higher during FU. In addition, the median [Q1 ; Q3] time to reach a response in CP pts was of 4.8 months [3.1 ; 10.9]. At the time of the interim analysis, 72.3% of pts presented at least one adverse event (AE), including 57.1% of pts with events related to PON which justified stopping treatment in 27.7% of cases. The event was considered as related and serious for 13.3% (16/120) of pts.
Conclusion: In a population of pts highly representative of TKI-resistant French CML pts, we report here a response improvement for 60% of CP-CML pts (MMR achievement) and 66.7% of AP/BP pts (CHR achievement). Ponatinib treatment dose was rapidly adapted (within 3 months) whatever the initial treatment dose limiting the number and severity of the AEs.
Disclosures
Cayssials:Novartis: Honoraria; Incyte Biosciences: Honoraria; Pfizer: Honoraria; BMS: Honoraria. Etienne:novartis: Honoraria; bms: Honoraria; pfizer: Honoraria; incyte: Honoraria. Turhan:Novartis: Consultancy, Honoraria; Incyte Biosciences: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Rousselot:Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Pfizer: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Takeda: Consultancy. Coiteux:novartis: Honoraria; incyte: Honoraria; pfizer: Honoraria; bms: Honoraria. Berger:incyte: Honoraria. Huguet:novartis: Honoraria; incyte: Honoraria; bms: Honoraria; amgen: Honoraria; pfizer: Honoraria; jazz pharma: Honoraria. Guerci-Bresler:incyte: Honoraria; bms: Honoraria; amgen: Honoraria; pfizer: Honoraria; novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal